January 25, 2022
Landmark Prospective Evidence on Posterior Approach to SI Joint Fusion Reflects Improved Pain Scores at 6 & 12 Months
SI joint fusion with PainTEQ’s LinQ implant shows promising results for patients with sacroiliitis.

TAMPA, Fla., Dec. 17, 2021 /PRNewswire/ — Preliminary data from the landmark SI joint fusion study of Tampa-based medical device company, PainTEQ, reveals that a posterior approach to SI joint fusion with the LinQ implant improved pain scores over a period of three to 12 months.
The Single-arm, Multicenter, Prospective, Clinical Study on a Novel Minimally Invasive Posterior Sacroiliac Fusion Device (SECURE) study is the first-ever comprehensive evaluation of PainTEQ’s posterior approach. Initial data on 57 patients at six months showed zero serious adverse events (SAE), suggesting that LinQ is safer than the traditional lateral approach, which reported 15.1% SAE incidence.
“We are very encouraged by the interim evidence for the efficacy, safety, and durability of LinQ,” said Dawood Sayed M.D., Professor of Anesthesiology and Pain Medicine at The University of Kansas Medical Center and one of the study’s key investigators. “The data – in terms of pain relief, functional improvement, and safety – support the use of LinQ as an important treatment within the algorithm of patients suffering from chronic sacroiliac joint disease as well as being grounded in high-quality prospective evidence.”
Preliminary data on the SECURE study also reflects a significant improvement of VAS scores, a unidimensional measure of pain intensity. Patients treated with LinQ experienced a reduction in pain at the three, six and 12-month follow-up, reinforcing the durability of the LinQ procedure (See Figure 1).
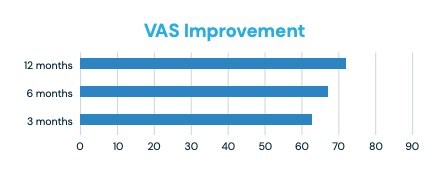
Figure 1
“In our planned interim analysis of our multicenter study, the six-month outcome has unparalleled safety and non-inferior results to the lateral fusion approach, as compared to what is published in peer-reviewed literature,” said co-investigator Jason Pope, M.D. “This represents the largest treatment arm for prospectively acquired sacroiliac joint fusion to date, across all approaches, and we look forward to the completion of the study.”
The researchers expect to complete SECURE study enrollment by the second quarter of 2022.
“Our LinQ system has experienced strong adoption across the United States this past year, and we are confident in providing more access to the wonderful physicians and nurses focused on the very best SI joint system in this new year,” said Michael Enxing, President of PainTEQ.
PainTEQ hopes the results from this landmark study will encourage more physicians to identify the SI joint as a pain generator and help patients suffering from SI joint dysfunction with a durable, evidence-backed therapy such as the LinQ system.
About PainTEQ: PainTEQ was built to bring interventional procedures to the market. Working with pain management specialists to help reduce and eliminate SI joint dysfunction, PainTEQ’s LinQ therapy aims to immediately provide clinical benefits to individuals living with incapacitating lower back pain through a minimally invasive outpatient procedure.
About LinQ: The LinQ SI Joint Stabilization System provides patients with a minimally invasive option to combat pain. After a thorough diagnostic process, physicians may help alleviate, and in many cases eliminate, chronic pain by placing a single LinQ allograft into the SI Joint. With its large graft window, this single implant helps create an ideal environment for long-term fusion.
###
Contact Information:
Kelly Citron, Marketing Manager
Kelly.Citron@painteq.com
855.248.PAIN
